Other Options
| Order Qty |
Price | Qty for Discount |
Discount Price |
Total Savings |
||||
|---|---|---|---|---|---|---|---|---|

|
NewPath Learning Properties & States of Matter Learning Guide Item # 21-2010-01 |
|
|
$9.95 | ||||

|
NewPath Learning Atoms & Chemical Bonding Learning Guide Item # 21-2010-08 |
|
|
$9.95 | ||||

|
NewPath Learning Chemical Reactions Learning Guide Item # 21-2010-07 |
|
|
$9.95 | ||||

|
NewPath Learning Energy: Forms & Changes Learning Guide Item # 21-2010-02 |
|
|
$9.95 | ||||

|
NewPath Learning Forces & Motion Learning Guide Item # 21-2010-03 |
|
|
$9.95 | ||||

|
NewPath Learning Work, Power & Simple Machines Learning Guide Item # 21-2010-05 |
|
|
$9.95 | ||||

|
NewPath Learning Sound Learning Guide Item # 21-2010-04 |
|
|
$9.95 | ||||

|
NewPath Learning Light & Optics Learning Guide Item # 21-2010-09 |
|
|
$9.95 | ||||

|
NewPath Learning Electricity & Magnetism Learning Guide Item # 21-2010-10 |
|
|
$9.95 | ||||
Additional Details
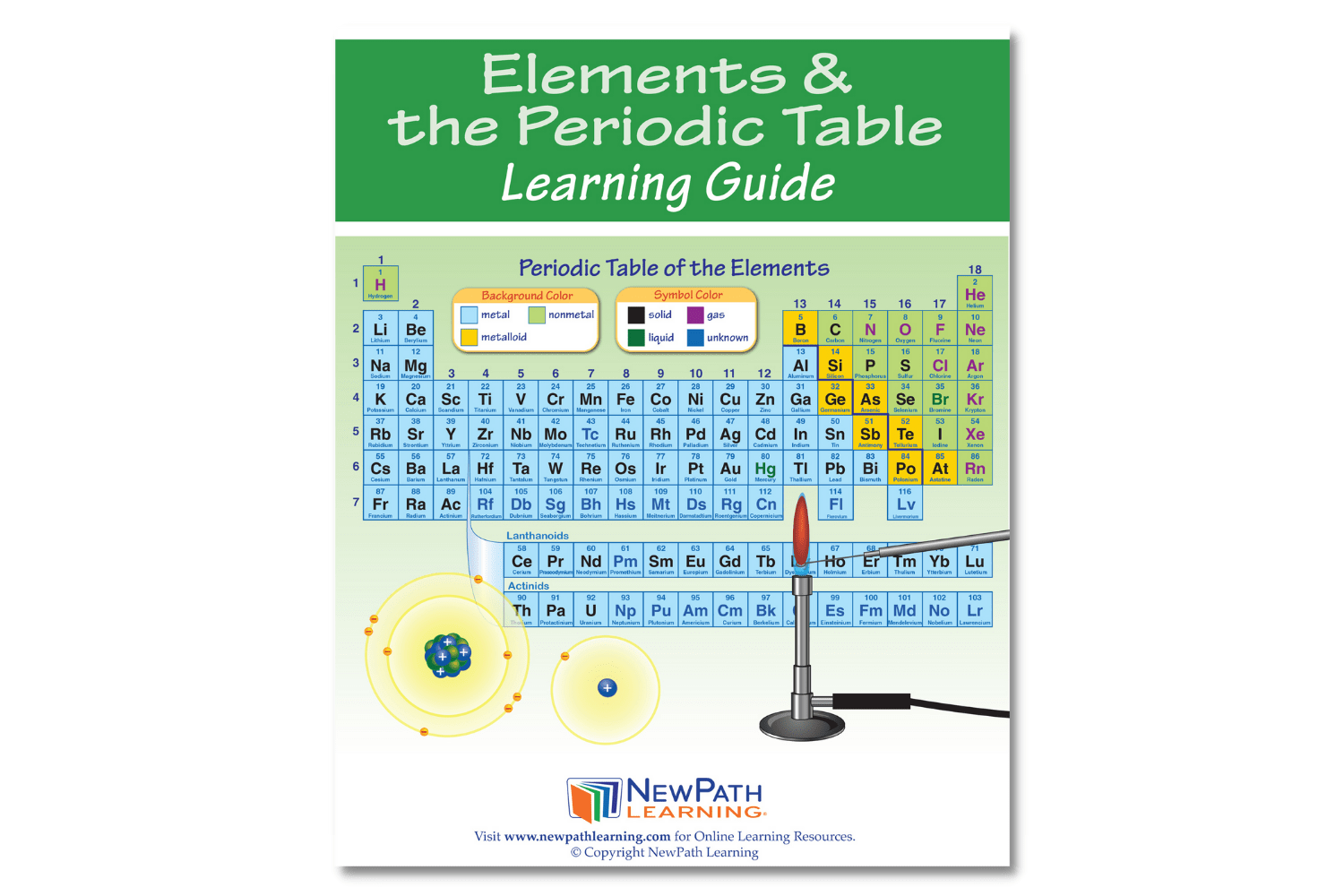
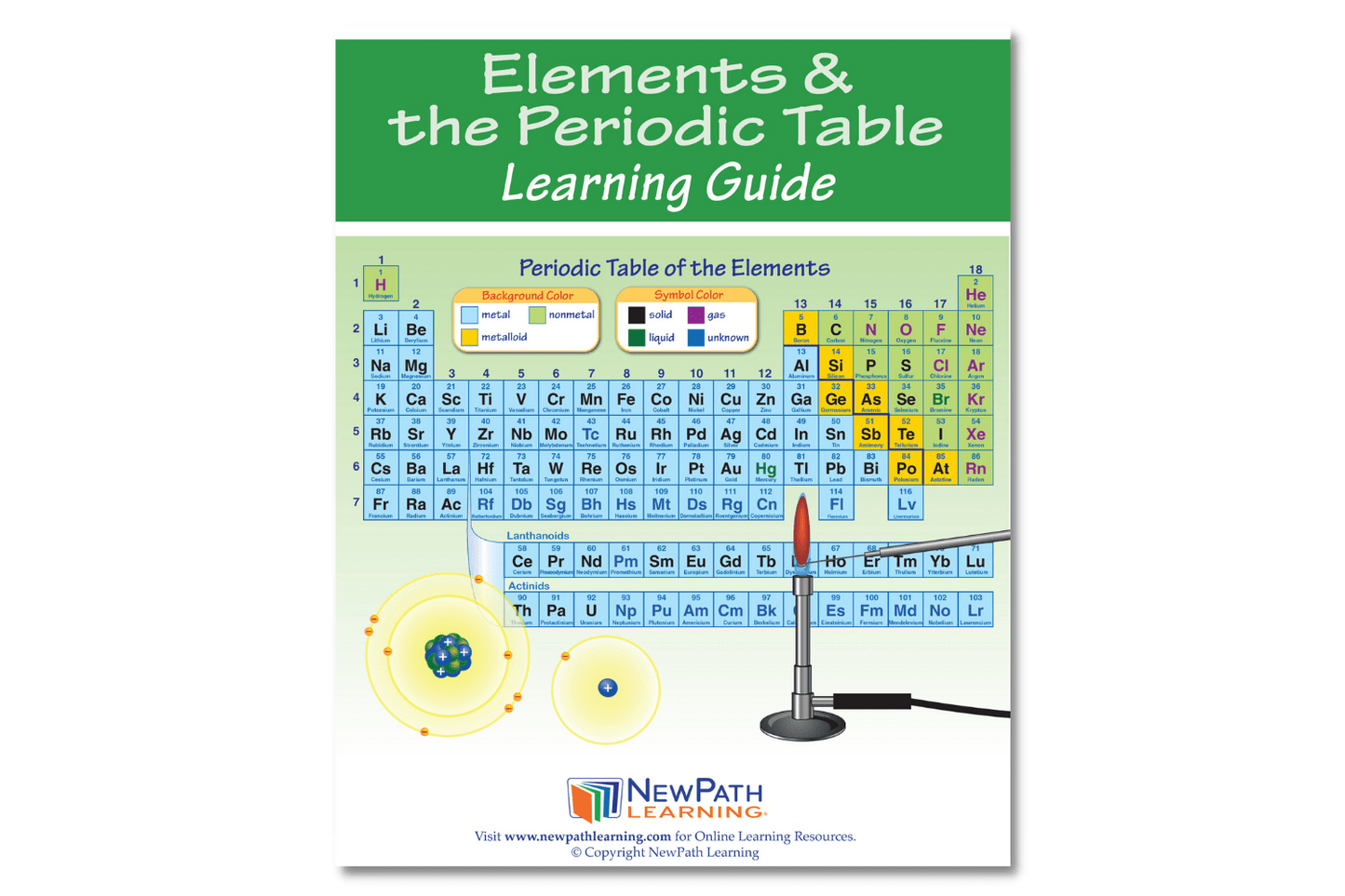
The NewPath Learning Elements & the Periodic Table Learning Guide turns the complex topic into an easy-to-learn, visually captivating, and engaging guide page by page! In the NewPath Learning Guide, you'll find self-directed readings, easy-to-follow illustrated explanations, guiding questions, inquiry-based activities, a lab investigation, key vocabulary review, and assessment review questions along with a post-test. The NewPath Learning Guide allows students to write directly in them and is designed for Grades 6-10.
This 44 page NewPath Learning Elements & the Periodic Table Learning Guide covers the following topics:
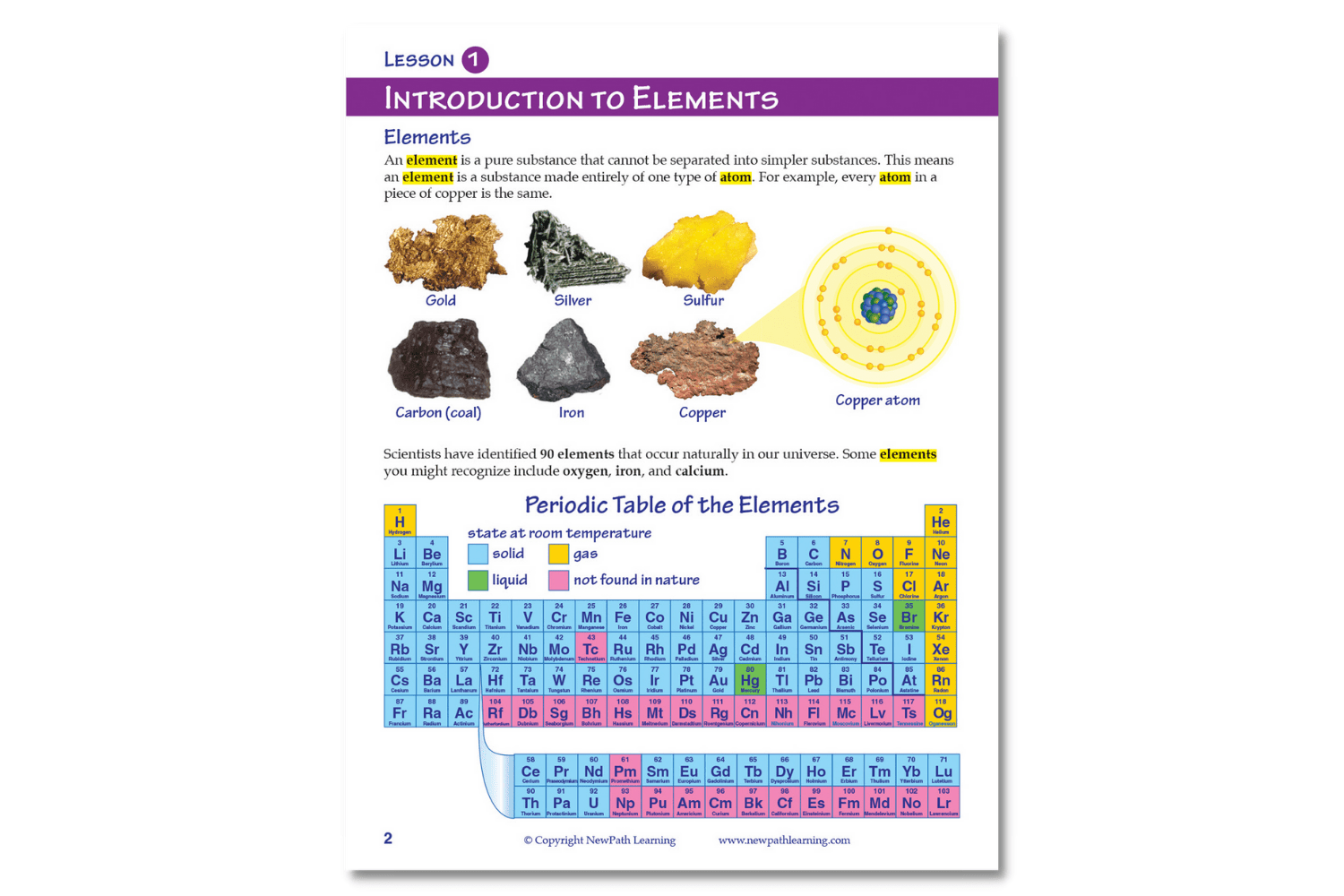
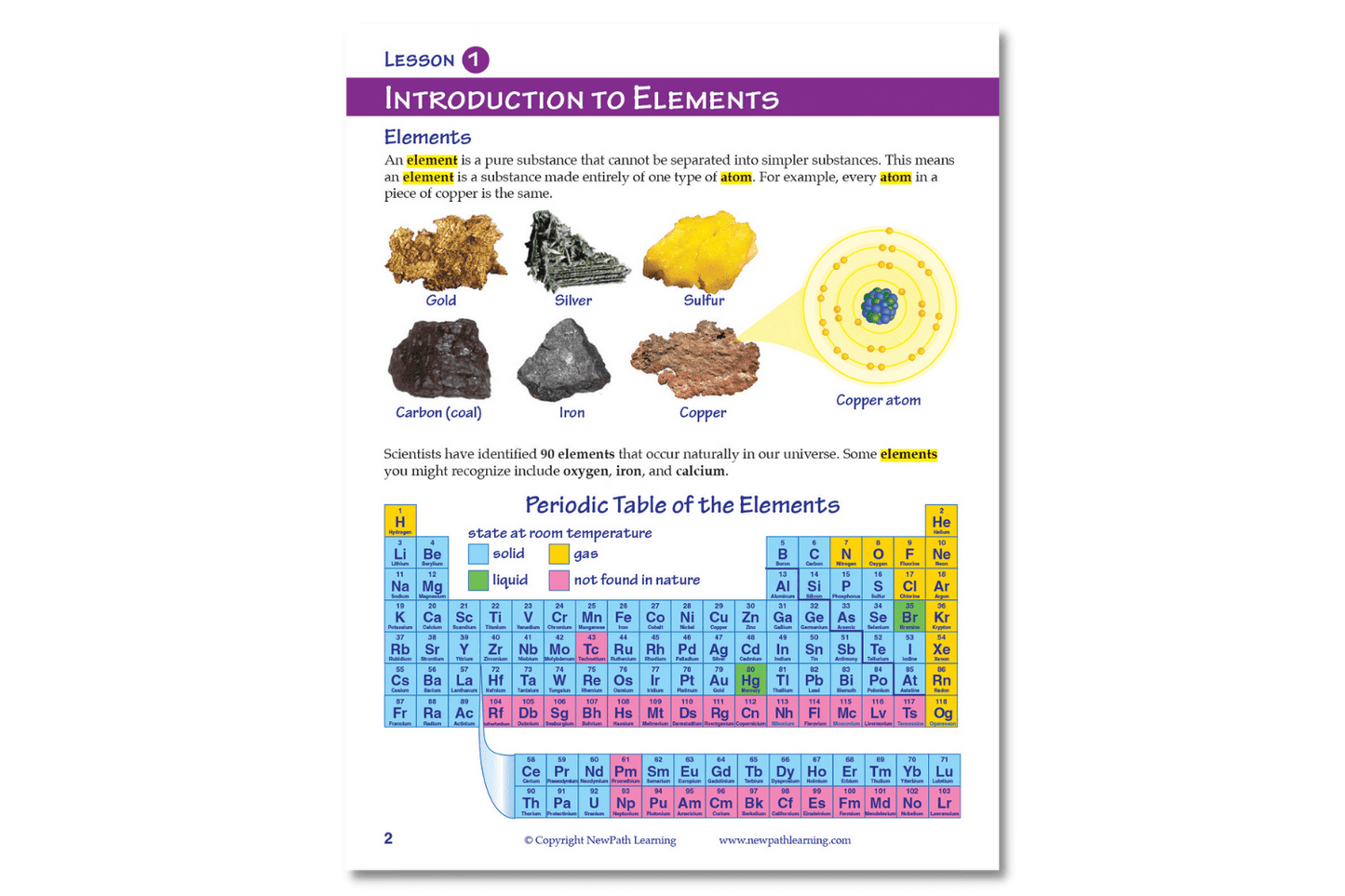
- Introduction to Elements
- Atomic Structure
- Classes of Elements – Metals
- Classes of Elements – Metalloids
- Classes of Elements – Nonmetals
- The Periodic Table
- Groups on the Periodic Table
- Groups on the Periodic Table
- Flame Test – Identifying Elements
- Vocabulary Review
Be confident knowing the NewPath Learning Guide covers Middle School Next Generation Science Standards.
Standards
Middle School (Grades 6, 7, 8) NGSS Correlations
| STRAND | NGSS.MS-PS. | PHYSICAL SCIENCE |
| TITLE | MS-PS1. | Matter and Its Interactions - Students who demonstrate understanding can: |
| PERFORMANCE EXPECTATION / FOUNDATION | MS-PS1.DCI. | Disciplinary Core Ideas |
| ELEMENT | PS1.A: | Structure and Properties of Matter |
| INDICATOR | PS1.A:1. | Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. (MS-PS1-1) |
| INDICATOR | PS1.A:2. | Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. (MS-PS1-2), (MS-PS1-3) |
Grade: 7 - Adopted 2013
| STRAND | NGSS.MS-PS. | PHYSICAL SCIENCE |
| TITLE | MS-PS1. | Matter and Its Interactions - Students who demonstrate understanding can: |
| PERFORMANCE EXPECTATION / FOUNDATION | MS-PS1.DCI. | Disciplinary Core Ideas |
| ELEMENT | PS1.A: | Structure and Properties of Matter |
| INDICATOR | PS1.A:1. | Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. (MS-PS1-1) |
| INDICATOR | PS1.A:2. | Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. (MS-PS1-2), (MS-PS1-3) |
High School (Grades 9) NGSS Correlations
| STRAND | NGSS.HS-PS. | PHYSICAL SCIENCE |
| TITLE | HS-PS1. | Matter and Its Interactions - Students who demonstrate understanding can: |
| PERFORMANCE EXPECTATION / FOUNDATION | HS-PS1-1. | Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms. |
| STRAND | NGSS.HS-PS. | PHYSICAL SCIENCE |
| TITLE | HS-PS1. | Matter and Its Interactions - Students who demonstrate understanding can: |
| PERFORMANCE EXPECTATION / FOUNDATION | HS-PS1.SEP . | Science and Engineering Practices |
| ELEMENT | HS-PS1.SEP .4. | Constructing Explanations and Designing Solutions - Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. |
| INDICATOR | HS-PS1.SEP .4.2. | Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future. (HS-PS1-2) |
| STRAND | NGSS.HS-PS. | PHYSICAL SCIENCE |
| TITLE | HS-PS1. | Matter and Its Interactions - Students who demonstrate understanding can: |
| PERFORMANCE EXPECTATION / FOUNDATION | HS-PS1.DCI. | Disciplinary Core Ideas |
| ELEMENT | PS1.A: | Structure and Properties of Matter |
| INDICATOR | PS1.A:1. | Each atom has a charged substructure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons. (HS-PS1-1) |
| INDICATOR | PS1.A:2. | The periodic table orders elements horizontally by the number of protons in the atom’s nucleus and places those with similar chemical properties in columns. The repeating patterns of this table reflect patterns of outer electron states. (HS-PS1-1), (HS-PS1-2) |